New year, New series!
Happy new year, my friends! I hope you are having a great new year so far! With the new year, comes a new series on Biochemisty for Life! I am planning to start a series called: The enzyme of the month. Every month, I will write a blog describing various enzymes and their contribution to the normal functioning of a biological system. Just as an introduction blog, I will like to describe what enzymes are.
For starters, enzyme
names end with the suffix “-ase.” For example, lipase, an enzyme that breaks
down lipids (fats), DNA Polymerase, which has a major role in DNA replication,
and topoisomerase, enzymes that bring about the winding and unwinding of the
DNA. The enzymes mentioned here end with the suffix “ase.” Biologists are very
serious about naming their beloved macromolecule. Why are these enzymes so
important? The answer lies in the fact that enzymes are biological catalysts.
Without these enzymes, carrying out the metabolic processes in the body would become
difficult because of the high activation energy. Enzymes, thus, lower the
activation energy of a particular chemical reaction. There are various methods
that enzymes apply for lowering the activation energy. One of these methods is
changing the conformation of the reactants. Because structure defines function
in biology, a small conformational change in the reactants can help with
initiating the chemical reaction. You must be wondering, why on Earth we
require enzymes for lowering the activation energy in the biological systems?
Without the ability to lower the activation energy, the chemical reactions in
living organisms would be a problem. In biological systems, high activation
energy can be unsuitable, especially, because the energy is in the form of heat.
High temperature can lead to a change in structure, and as mentioned earlier,
structure defines function in biology. A slight structural change can lead to
damage to the function.
Let us now explore the structure of the enzyme. Enzymes are proteins which means their structure comprises amino acids. The site where the enzymes bind to their substrates (reactants) is referred to as an active site. These active sites are very particular about what substrates they bind to. Therefore, an enzyme has a specific role in a biological system. Two models describe the enzyme-substrate binding:
A. Lock and Key model: The enzyme and substrate binding is perfect. There is no conformational change observed in the enzyme for the substrate to bind to the active site.
B. Induced Fit model: The enzyme has to undergo a slight structural change for the substrate to bind to the active site1. (Please refer to the image below)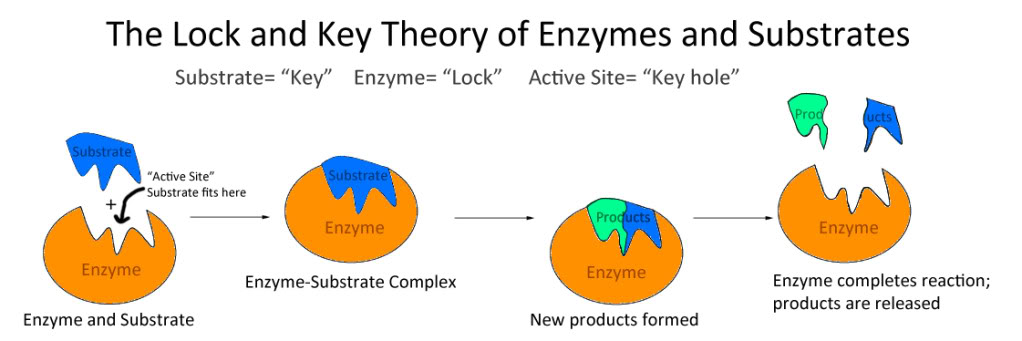
There are many natural factors that regulate the activity
of the enzymes including pH, temperature, substrate saturation, and enzyme
concentration. All the enzymes have a typical pH and temperature range where
they optimally perform their tasks. If the enzyme experiences changes in the pH
or temperature, the structure of the enzyme, and its function can be compromised.
Moreover, if there are more substrates than the enzymes, the rate of the reactions lower than the normal. The body, therefore, has a system that “communicates” the
imbalance in the ratio between substrates and enzymes to the nucleus to induce
gene expression. Cellular communication is a rather complex topic that is a
discussion for the other day! These are natural factors that can affect the
reaction rates carried out by the enzymes.
There can be competitive and noncompetitive inhibition
that can affect the rate of the enzymes. Competitive inhibitors2
bind to the active site of the enzymes and “compete” with the substrates of the
enzymes. The binding of the inhibitors can cause a conformational change in the
active sites which has the ability to permanently block the enzyme activity.
Whereas non-competitive substrates bind to the allosteric site 3 of
the enzymes that temporarily blocks the enzyme activity. This allows for saving the
energy of the cells when the enzyme activity is not needed.
On an ending note on this introductory blog, I would like to mention that I absolutely respect the value of enzymes. With this
new year series, I hope to cultivate this respect for the enzymes within my readers. Please stay tuned for more enzyme related blogs!
Footnotes:
1. 1. Even though structure defines function in biology, this slight conformational change is needed for the enzymes to carry out their tasks.
2. 2. An example of competitive inhibitors is cyanide which blocks the
enzymes participating in cellular respiration. Hence, the cells cannot produce
energy leading to dangerous consequences.
3. 3. A binding site that is different than an active site on an enzyme. A substrate
that induces or inhibits the enzyme activity binds to this site to regulate the activity of the enzyme.
Works Cited:
Enzymes - an overview | ScienceDirect Topics. (2011). Sciencedirect.com. https://www.sciencedirect.com/topics/neuroscience/enzymes
6.2D: Activation Energy. (2018, July 10). Biology LibreTexts. https://bio.libretexts.org/Bookshelves/Introductory_and_General_Biology/Book%3A_General_Biology_(Boundless)/6%3A_Metabolism/6.2%3A_Potential_Kinetic_Free_and_Activation_Energy/6.2D%3A_Activation_Energy

